Courtesy : ctahr.hawaii.ed
Water and soil
In nutrient management, a proper balance between soil water and soil air is critical since both water and air are required by most processes that release nutrients into the soil. Soil water is particularly important in nutrient management. In addition to sustaining all life on Earth, soil water provides a pool of dissolved nutrients that are readily available for plant uptake. Therefore, it is important to maintain proper levels of soil moisture.
Soil water is important for three special reasons:
- The presence of water is essential for the all life on Earth, including the lives of plants and organisms in the soil.
- Water is a necessary for the weathering of soil. Areas with high rainfall typically have highly weathered soils. Since soils vary in their degree of weathering, it is expected that soils have been affected by different amounts of water.
- Soil water is the medium from which all plant nutrients are assimilated by plants. Soil water, sometimes referred to as the soil solution, contains dissolved organic and inorganic substances and transports dissolved nutrients, such as nitrogen, phosphorus, potassium, and calcium, to the plant roots for absorption.
The amount of water in the soil is dependent upon two factors:
- First, soil water is intimately related to the climate, or the long term precipitation patterns, of an area.
- Secondly, the amount of water in the soil depends upon how much water a soil may hold.
Soil water holding capacity
Before we discuss the capacity of soils to hold water, we must understand the concept of capillarity.
Capillarity
- Water molecules behave in two ways:
- Cohesion Force: Because of cohesion forces, water molecules are attracted to one another. Cohesion causes water molecules to stick to one another and form water droplets.
- Adhesion Force: This force is responsible for the attraction between water and solid surfaces. For example, a drop of water can stick to a glass surface as the result of adhesion.
- Water also exhibits a property of surface tension:
- Water surfaces behave in an unusual way because of cohesion. Since water molecules are more attracted to other water molecules as opposed to air particles, water surfaces behave like expandable films. This phenomenon is what makes it possible for certain insects to walk along water surfaces.
- Capillary Action:
- Capillary action, also referred to as capillary motion or capillarity, is a combination of cohesion/adhesion and surface tension forces.
- Capillary action is demonstrated by the upward movement of water through a narrow tube against the force of gravity.
- Capillary action occurs when the adhesive intermolecular forces between a liquid, such as water, and the solid surface of the tube are stronger than the cohesive intermolecular forces between water molecules.
- As the result of capillarity, a concave meniscus (or curved, U-shaped surface) forms where the liquid is in contact with a vertical surface.
- Capillary rise is the height to which the water rises within the tube, and decreases as the width of the tube increases. Thus, the narrower the tube, the water will rise to a greater height.

Figure 3. Capillary rise in tubes of varied widths. This picture demonstrates the phenomenon of capillary rise. As you can see, the liquid rises to the greatest height in the narrowest tube (at far right), whereas capillary rise is lowest in the widest tube (at far left). Although easily demonstrated by simple experiments using tubes, capillary action occurs in soils. Smaller pores that exist in finely-textured soils have a greater capacity to hold and retain water than coarser soils with larger pores.
Source: http://www.wtamu.edu/~crobinson/SoilWater/capillar.html
Capillary action is the same effect that causes porous materials, such as sponges, to soak up liquids.
- Capillarity is the primary force that enables the soil to retain water, as well as to regulate its movement.
- The phenomenon of capillarity also occurs in the soil. In the same way that water moves upwards through a tube against the force of gravity; water moves upwards through soil pores, or the spaces between soil particles.
- The height to which the water rises is dependent upon pore size. As a result, the smaller the soil pores, the higher the capillary rise.
- Finely-textured soils, like in Maui, typically have smaller pores than coarsely-textured soils. Therefore, finely-textured soils have a greater ability to hold and retain water in the soil in the inter-particle spaces. We refer to the pores between small clay particles as micropores. In contrast, the larger pore spacing between lager particles, such as sand, are called macropores.
- In addition to water retention, capillarity in soil also enables the upward and horizontal movement of water within the soil profile, as opposed to downward movement caused by gravity. This upward and horizontal movement occurs when lower soil layers have more moisture than the upper soil layers and is important because it may be absorbed by roots.
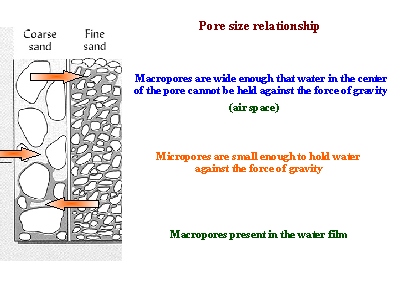
Figure 4. This picture shows how more water may be held between finer particles against the force of gravity, as compared to coarser particles. As a result, finer-textured soils have greater water holding capacities.
Source: http://forest.mtu.edu/classes/fw3330/water_2004/slide19.html
Water holding capacity
Since water is held within the pores of the soil, the water holding capacity depends on capillary action and the size of the pores that exist between soil particles. Sandy soils have large particles and large pores. However, large pores do not have a great ability to hold water. As a result, sandy soils drain excessively. On the other hand, clayey soils have small particles and small pores. Since small pores have a greater ability to hold water, clayey soils tend to have high water holding capacity.


